
Atulya Iyengar
Assistant Professor
- atulya.iyengar@ua.edu
- 2328 Science and Engineering Complex (SEC)
- Not Accepting Students
Education
- PhD, Neuroscience, University of Iowa
Research Interests
Proper brain function requires the concerted action of a diverse array of gene products. Our broad research interests center on how genes which regulate neuronal excitability (e.g. ion channels) and/or neurotransmission (e.g. neurotransmitter receptors) interact with one another and with the extrinsic environmental factors (e.g. diet, stress, temperature) to shape normal and aberrant nervous system activity. We utilize the fruit fly, Drosophila melanogaster, as a model organism, and employ both classical and transgenic approaches to precisely manipulate the genome. In these single- and double-mutant flies, we correlate changes in neuronal structure and function with alterations in motor circuit activity patterns and the organization of behavioral repertoires. Findings from our work provide basic insights on the functional organization of the Drosophila nervous system and often have implications in understanding the etiology neurological disease.
Videos:
Electrically evoked seizures in flies compared to flight and walking/rest activity modes. High-frequency electroconvulsive stimulation across the head induces hyper-synchronization in the fly brain. A newly developed local field potential (LFP) technique monitors brain activity, while microelectrodes in the thorax record flight muscle motor unit spiking (L DLM and R DLM). A microphone below the fly picks-up wing sounds. Notice the large amplitude LFP oscillations during the seizure which are absent in flight and rest modes. For more information see Iyengar & Wu, 2021 J Neurogenet. https://doi.org/10.1080/01677063.2021.1950714
Pharmacological seizure induction in Drosophila. We recently developed a drug injection technique to systemically introduce pro- or anti-convulsants in intact behaving flies. In this video we inject picrotoxin (PTX) a GABAA receptor antagonist which suppresses inhibitory neurotransmission and leads to convulsions. Blue #1 dye visualizes drug circulation, while video and electrophysiological recordings monitor convulsions. For more information, see Lee, Iyengar & Wu, 2019. J Neurogenet. https://doi.org/10.1080/01677063.2019.1581188
Automated video tracking of flies carrying a mutation in the vitamin B6 metabolism gene PNPO linked with neonatal epileptic encephalopathy (R95H). Note the ataxic locomotion phenotype (above) which is ameliorated by feeding active vitamin B6 (PLP, below). For more information see Chi et al., 2021 Natl. Acad. Sci. USA https://doi.org/10.1073/pnas.2115524119
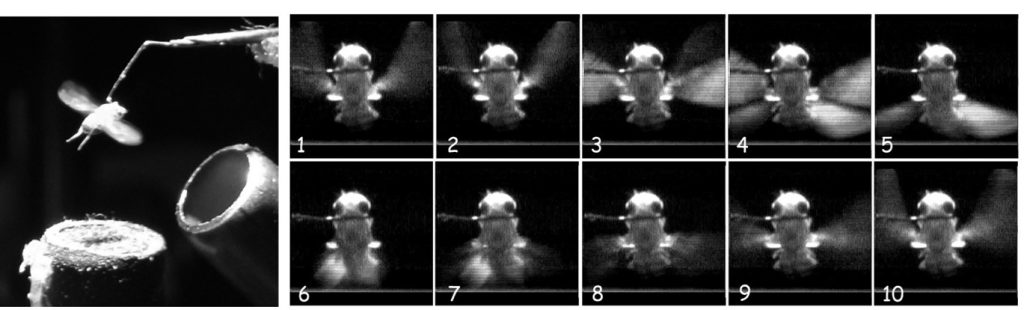 Still: High speed videography of wing-beat kinematics in Drosophila. (Left) Tethered fly preparation. (Right) High speed video of wing position during phases of the wing-beat.
Still: High speed videography of wing-beat kinematics in Drosophila. (Left) Tethered fly preparation. (Right) High speed video of wing position during phases of the wing-beat.
 Still: High speed videography of wing-beat kinematics in Drosophila. (Left) Tethered fly preparation. (Right) High speed video of wing position during phases of the wing-beat.
Still: High speed videography of wing-beat kinematics in Drosophila. (Left) Tethered fly preparation. (Right) High speed video of wing position during phases of the wing-beat.
Selected Publications
- *Iyengar, A., *Chakraborty, T., Goswami, S., Wu, C.-F., Siddiqi, O. (2010). Post-eclosion odor experience modifies olfactory receptor coding in Drosophila. Proc. Natl. Acad. Sci. USA, 107(21), 9855-9860. https://doi.org/10.1073/pnas.1003856107
- *Iyengar, A., *‡Imoehl, J., Ueda, A., ‡Nirschl, J., Wu, C.-F. (2012) Automated quantification of locomotion, social interaction and mate preference in Drosophila mutants. J. Neurogenet. 26(3-4) 306-316. https://doi.org/10.3109/01677063.2012.729626
- *Ehaideb, S., *Iyengar, A., Ueda, A., Iacobucci, G., Cranston, C., Bassuk, A., Gubb, D., Axelrod, J., Gunawardena, S., Wu, C.-F., Manak, J. (2014). prickle modulates polarity and axonal transport to ameliorate seizures in flies. Proc. Natl. Acad. Sci. USA 111(30), 11187-11192. https://doi.org/10.1073/pnas.1403357111
- Iyengar, A., Wu, C.-F., (2014). Flight and seizure motor patterns in Drosophila mutants: Simultaneous acoustic and electrophysiological recordings of wing beats and flight muscle activity. J. Neurogenet., 28(3-4) 316-328. https://doi.org/10.3109/01677063.2014.957827
- *Lee, J., *Iyengar, A., Wu, C.-F. (2019) Distinctions among electroconvusion- and proconvulsant-induced seizure discharges during flight and grooming: quantitative spike pattern analysis in Drosophila flight muscles. J. Neurogenet. 33(2) 125-142. https://doi.org/10.1080/01677063.2019.1581188
- Kasuya, J., Iyengar, A., Chen, H.-L., Lansdon, P., Wu, C.-F., Kitamoto, T. (2019) Milk whey substantially suppresses seizure-like phenotypes of paraShu, a Drosophila voltage-gated sodium channel mutant. J. Neurogenet. 33(2), 164-178. https://doi.org/10.1080/01677063.2019.1597082
- Iyengar, A., Wu, C.-F. (2021) Fly seizure EEG: field potential activity in the Drosophila brain. J. Neurogenet., 35(3) 295-305 . https://doi.org/10.1080/01677063.2021.1950714
- *Iyengar, A., *Ruan, H., Wu, C.-F. (2022). Distinct aging-vulnerable trajectories of motor circuit functions in oxidation- and temperature-stressed Drosophila. eNeuro 9(1) 0433-21.221 https://doi.org/10.1523/ENEURO.0443-21.2021 [Cover Feature]
- *Chi, W., *Iyengar, A., Fu, W., Liu, W., ‡Berg, A., Wu, C.-F., Zhuang, X. (2022) Diet modifies allele-specific phenotypes in Drosophila carrying epilepsy-associated PNPO variants. Proc. Natl. Acad. Sci. USA. 119(9) e2115524119. https://doi.org/10.1073/pnas.2115524119