
Matthew Jenny
Associate Professor
Chair of Program Assessment Committee
- mjjenny@ua.edu
- (205) 348-8225
- 3318 Science and Engineering Complex (SEC)
- Website
- Not Accepting Students
Education
- Postdoctoral research, Woods Hole Oceanographic Institution
- Ph.D., Molecular and Cellular Biology and Pathobiology, Medical University of South Carolina, 2005
Research Interests
My primary interests are in the field of functional genomics and molecular toxicology. I am broadly interested in factors influencing the evolution and control of transcriptional regulatory networks, as well as mechanisms of molecular adaptation to environmental stressors and ‘systems biology’ approaches to constructing genome-wide models of genetic regulation of environmental and ecological responses.
Evolution of transcription regulatory networks
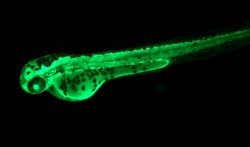 My lab studies the interactive roles of several transcription factors (MTF-1, NRF2, HIF-1a, AHR) that have dual roles in basic developmental processes and also serve as master regulators of a variety of different stress-response pathways. We are interested in the role of these transcription factors in regulating both protein coding genes and microRNA genes. Zebrafish is the main model organism we use for this research because results obtained in this system have direct relevance to humans. A key advantage of zebrafish is the external development of nearly transparent embryos which facilitates direct observation of all stages of embryonic development. We use a variety of molecular approaches to accomplish our research: quantitative PCR, microarray gene expression profiling, targeted gene knockdown with antisense morpholinos, creation of transient and stable transgenics, promoter/enhancer functional analysis, and mapping of regulatory elements.
My lab studies the interactive roles of several transcription factors (MTF-1, NRF2, HIF-1a, AHR) that have dual roles in basic developmental processes and also serve as master regulators of a variety of different stress-response pathways. We are interested in the role of these transcription factors in regulating both protein coding genes and microRNA genes. Zebrafish is the main model organism we use for this research because results obtained in this system have direct relevance to humans. A key advantage of zebrafish is the external development of nearly transparent embryos which facilitates direct observation of all stages of embryonic development. We use a variety of molecular approaches to accomplish our research: quantitative PCR, microarray gene expression profiling, targeted gene knockdown with antisense morpholinos, creation of transient and stable transgenics, promoter/enhancer functional analysis, and mapping of regulatory elements.
Environmental and ecological genomics
My lab works with a variety of freshwater and marine fish and invertebrates to study the evolution of metallothionein proteins, metal-binding proteins responsible for essential metal homeostasis and heavy metal detoxification. We have broad interests in molecular mechanisms which regulate metal homeostasis and convey metal tolerance, as well as mechanisms for ameliorating oxidative stress related to environmental pressures. We also utilize molecular and genomic techniques to investigate mechanisms of defense and tolerance in response to other environmental stressors (natural and anthropogenic). The incorporation of genomic-based research into traditional ecological models allows us to study and understand the physiological processes of organisms and their adaptation to specific ecological niches at the most fundamental levels.
Selected Publications
- Holowiecki, B. O’Shields, M.J. Jenny (2017). Spatiotemporal expression and transcriptional regulation of heme oxygenase and biliverdin reductase genes in zebrafish (Danio rerio) suggest novel roles during early developmental periods of heightened oxidative stress. Comparative Biochemistry and Physiology – Part C: Toxicology and Pharmacology 191: 138-151.
- S.L. Payton, P. Johnson, M.J. Jenny (2016). Comparative physiology, biochemical, and molecular thermal stress response profiles for two Unionid freshwater mussel species. Journal of Experimental Biology, 219: 3562-3574.
- Holowiecki, B. O’Shields, M.J. Jenny (2016). Characterization of heme oxygenase and biliverdin reductase gene expression in zebrafish (Danio rerio): basal expression and response to pro-oxidant exposure. Toxicology and Applied Pharmacology, 311: 74-87.
- M.J. Jenny, S.L. Payton, D.A. Baltzegar, J.D. Lozier (2016). Phylogenetic comparison of molluscan metallothioneins: Evolutionary insight from Crassostrea virginica. Journal of Molecular Evolution 83(3-4): 110-125.
- M.J. Jenny, W.C. Walton, S.L. Payton, J.H. Powers, R.H. Findlay, B. O’shields, K. Diggins, M. Pinkerton, D. Porter, D.M. Crane, J. Tapley, Charles Cunninham (2016). Transcriptomic evaluation of the American Oyster, Crassostrea virginica, deployed during the Deepwater Horizon Oil Spill: Evidence of an active hydrocarbon response pathway. Marine Environmental Research 120: 166-181.
- O’Shields, A.G. McArthur, A. Holowiecki, M. Kamper, J. Tapley, M.J. Jenny (2014). Inhibition of endogenous MTF-1 signaling in zebrafish embryos identifies novel roles for MTF-1 in development. BBA Molecular Cell Research 1843(9): 1818-1833.
- A.M. Tarrant, A.M. Reitzel, C.K. Kwock, M.J. Jenny (2014). Activation of the cnidarian oxidative stress response by ultraviolet light, polycyclic aromatic hydrocarbons and crude oil. Journal of Experimental Biology 217(9): 1444-1453.
- M.J. Jenny, Neelakanteswar Aluru, Mark E. Hahn (2012). Effects of short-term exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on microRNA expression in zebrafish embryos. Toxicology and Applied Pharmacology 264(2): 262-273